New findings on Titan reinforce its astrobiological interest: active chemistry, clouds and methane rain
Data from three instruments aboard the James Webb Space Telescope offer fresh insights into Titan’s atmosphere and chemistry—among the most complex and stable in the solar system
One of the study’s key findings, involving participation from the Instituto de Astrofísica de Andalucía (IAA-CSIC), is the first direct detection of the methyl radical (CH₃) in Titan’s atmosphere, confirming major predictions about the moon’s rich organic chemistry
Like Earth, Titan—Saturn’s largest moon—has a nitrogen-rich atmosphere and weather systems that include clouds and rain. But unlike our planet, where water drives the climate, Titan’s cryogenic environment revolves around methane: it evaporates, forms clouds, falls as rain, and fills lakes and seas on the surface. This methane cycle, along with a complex web of organic chemistry, has been the subject of new discoveries that shed light on Titan’s fascinating atmosphere.
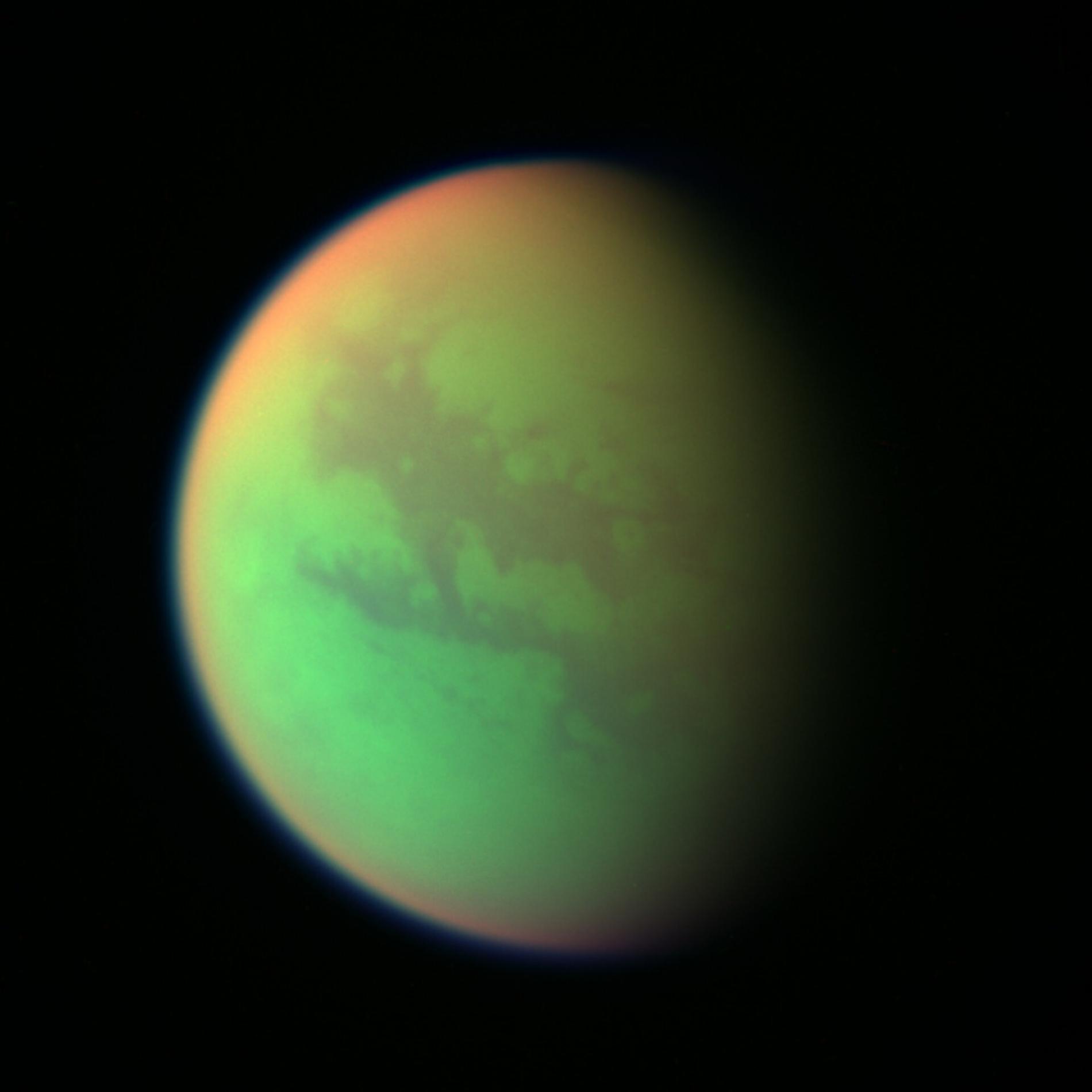
False-colour composite created from images taken during the closest flyby of the NASA/ESA/ASI Cassini spacecraft to Titan in 2025. Green represents the areas where Cassini can see the surface. Red represents the high areas of Titan's stratosphere. The blue along the outer edge of the moon represents the visible violet wavelengths where the upper atmosphere and the detached hazes are best seen. Credits: NASA/JPL/Space Science Institute.
An international team, including researchers from the Institute of Astrophysics of Andalusia (IAA-CSIC), has reported the first direct detection of the methyl radical (CH₃) in Titan’s atmosphere—a molecule composed of one carbon and three hydrogen atoms.
“The direct detection of CH₃ allows us to confirm what chemical models have long predicted: that Titan’s rich organic chemistry begins with a few highly reactive radicals, which we can now observe,” says Manuel López Puertas, a researcher at IAA-CSIC and co-author of the study.
The research also presents other key results, including the first complete profile of carbon monoxide (CO) in Titan’s atmosphere and early evidence of cloud convection in the moon’s northern hemisphere—currently in summer—above a region rich in lakes and seas. The results have been published in Nature Astronomy.
ADVANCES IN UNDERSTANDING TITAN’S COMPLEX CHEMISTRY
Shrouded in a thick yellowish haze, Titan remains one of the most intriguing astrobiological targets in the solar system. Much of this interest stems from its complex organic chemistry—that is, its wealth of carbon-based molecules. Organic molecules are the basic building blocks of life as we know it, and studying them in environments like Titan may offer valuable clues about the chemical pathways that led to life on early Earth.
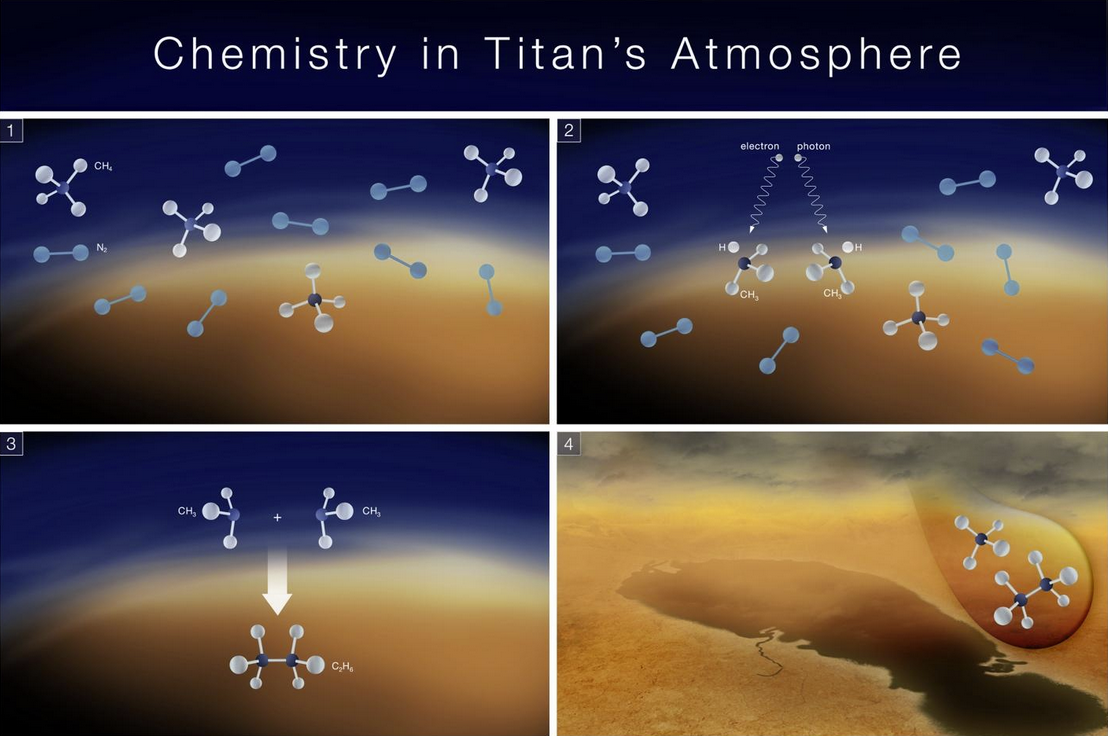
Key chemical process believed to occur in Titan's atmosphere. NASA's James Webb Space Telescope detected the methyl radical on Titan for the first time, providing a key missing piece to our understanding of Titan's chemical processes. Credits: NASA, ESA, CSA and Elizabeth Wheatley (STScI).
Methane (CH₄) plays a central role in Titan’s chemical activity. In its atmosphere, methane breaks apart under the influence of sunlight or energetic electrons from Saturn’s magnetosphere. These methane fragments can then react with each other or with other species to form more complex compounds, such as ethane (C₂H₆), and other organic molecules that may eventually settle on the surface.
Data from the James Webb Space Telescope’s Mid-Infrared Instrument (MIRI)—developed and operated by NASA, ESA, and CSA—provided a key piece of the puzzle: the first direct detection of the methyl radical (CH₃) in Titan’s atmosphere. This type of molecule, called a “radical” because it contains an unpaired electron, is created when methane breaks apart.
“Detecting CH₃ lets us witness chemistry in action in Titan’s atmosphere—not just the starting ingredients or end products, but also the intermediate steps of the process,” says Manuel López Puertas (IAA-CSIC).
This hydrocarbon-based chemistry not only reveals how organic compounds form in Titan’s atmosphere, but also has implications for the moon’s long-term evolution. When methane is broken down in the upper atmosphere, part of it transforms into other molecules that eventually settle on the surface. At the same time, some of the released hydrogen escapes into space. This means Titan’s methane supply is slowly being depleted—unless there is some internal source replenishing it.
“On Titan, methane is a resource that’s gradually being consumed. It may be replenished from the interior, seeping up through the crust over billions of years. But if that’s not the case, Titan’s methane will eventually run out, and the moon will turn into a world of dust and dunes,” warns Conor Nixon, researcher at NASA’s Goddard Space Flight Center and lead author of the study.
THE MYSTERY OF OXYGEN ON TITAN: COULD IT COME FROM BEYOND?
Another key finding came from the JWST’s NIRSpec instrument, a near-infrared spectrograph that allowed researchers to measure carbon monoxide (CO) concentrations from Titan’s surface up to the upper atmosphere—around 800 kilometers in altitude. Previous profiles had only reached 450 km. The new measurements confirm that CO concentration remains constant across all layers.
According to IAA-CSIC researcher Manuel López Puertas, “The fact that CO levels are constant from the troposphere to the exosphere suggests that the oxygen it contains isn’t produced on Titan—it must come from elsewhere.”
CO accounts for most of the oxygen present in Titan’s atmosphere, which is otherwise dominated by nitrogen, carbon, and hydrogen—making the origin of that oxygen a mystery. Current theories suggest that it may come from interplanetary dust or from material ejected by Enceladus, another of Saturn’s moons, in the form of OH/H₂O (hydroxyl/water) radicals and atomic oxygen (O).
The study also used an advanced model to estimate carbon dioxide (CO₂) levels, confirming previous measurements from the Cassini-Huygens mission—but the full picture of Titan’s oxygen cycle remains unresolved.
Together, the detection of CH₃ and the complete CO profile open new windows onto the atmospheric evolution of Titan and the processes that may have shaped early Earth–like environments.
“In the future, we hope to uncover more findings in JWST data,” says Manuel López Puertas (IAA-CSIC). “Thanks to its exceptional precision, high spectral resolution, and freedom from Earth’s atmospheric interference, the telescope may soon reveal trace compounds like nitriles or aromatic hydrocarbons in Titan’s atmosphere.”

Spectra of Titan measured by JWST. Above, NIRSpec. Bottom, MIRI. The main absorption and emission bands (the latter marked with an asterisk) of the main atmospheric compounds are shown, as well as the "windows" in which Titan's surface can be seen (near-transparent atmosphere, grey shaded regions). Credit: Nixon et al. (2025) Nature Astronomy. DOI: 10.1038/s41550-025-02537-3
TITAN’S WEATHER
On Titan, methane plays a meteorological role similar to that of water on Earth. It evaporates from lakes and seas, rises through the atmosphere, condenses into clouds, and falls again as cold, oily rain onto a solid surface. At Titan’s extremely low temperatures (−180 °C), water ice becomes as hard as rock.
The research team observed Titan in two campaigns—November 2022 and July 2023—combining data from JWST with observations from the ground-based W. M. Keck Observatory. Clouds were detected at mid and high northern latitudes, and appeared to rise over several days. While cloud convection had previously been observed in the southern hemisphere, this is the first time such activity has been documented in the north during summer.
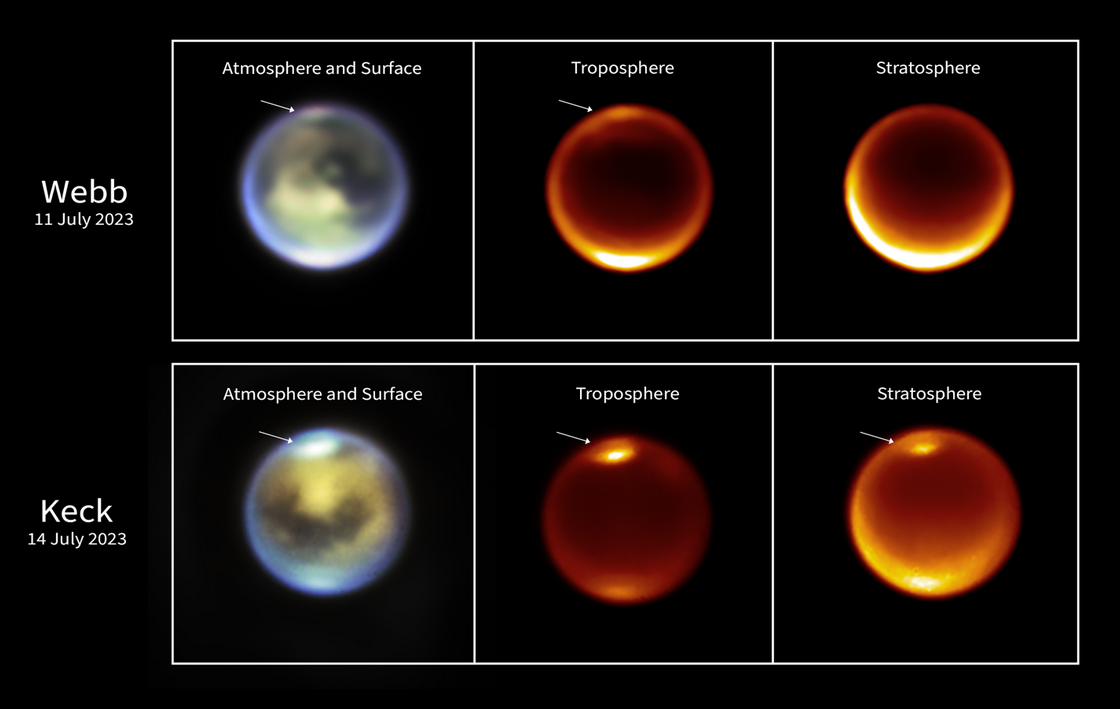
These images of Titan were taken by NASA's James Webb Space Telescope on 11 July 2023 (top row) and by the W.M. Keck ground-based observatories on 14 July 2023 (bottom row). They show methane clouds appearing at different altitudes in Titan's northern hemisphere. Credits: NASA, ESA, CSA, STScI and W.M. Keck Observatories.
“This finding is especially significant because most of Titan’s lakes and seas are located in its northern hemisphere, and the evaporation of these bodies is a key source of atmospheric methane,” explains the first author of the work.
On Earth, the troposphere—the atmospheric layer where weather occurs—extends to about 12 kilometers. On Titan, due to lower gravity, it stretches up to about 45 kilometers. Using various infrared filters, Webb and Keck were able to observe different depths in Titan’s atmosphere and estimate cloud altitudes, which appeared to increase over time. “Although we didn’t detect rain directly, the observations suggest conditions were favorable for precipitation,” concludes Conor Nixon (NASA-GSFC).
- ‘The atmosphere of Titan in late northern summer from JWST and Keck observations’
- https://www.nature.com/articles/s41550-025-02537-3
- Manuel López Puertas - puertas@iaa.es
Instituto de Astrofísica de Andalucía (IAA-CSIC)
Unidad de Divulgación y Comunicación
Amanda López – alm@iaa.es
Emilio García – garcia@iaa.es - 649 407 445 (vía whatssap)
Celia Navas - navas@iaa.es
https://www.iaa.csic.es
https://divulgacion.iaa.csic.es

